SELECTED RESEARCH INTERESTS
Positive and negative picokelvin temperatures
Nuclear magnetic ordering has been investigated at T>0 in copper and more
recently at T>0 and at T<0 in silver and rhodium. In copper and
silver, competition between the dipolar force and the conduction-electron
mediated exchange interaction leads, at T>0, to antiferromagnetic
structures. At negative spin temperatures, ferromagnetic order has been found
in silver. In this metal, the phase transitions occur at 560 pK and at
-1.9 nK, respectively. In rhodium, 280 pK and -750 pK have been reached;
these are the current world records of low temperatures.
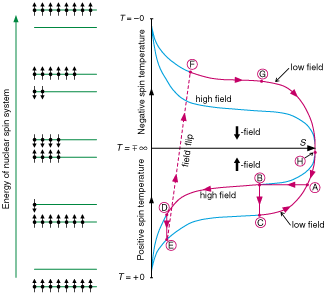
Fig. 8. Schematic illustration, on a temperature vs. entropy diagram,
of the procedure for cooling an assembly of silver or rhodium nuclei to
negative nanokelvin temperatures. At left, the energy level diagram of the
nuclear spins is shown.
(H -> A) Both nuclear stages, are cooled to Ti = 15 mK by
the dilution refrigerator and (A -> B) polarized in a magnetic field slowly
increased to Bi = 8 T.
(B -> C) The nuclei of the first stage, made of 20 mol of copper, are
adiabatically demagnetized to Bf = 100 mT, producing a low
temperature Tf = Ti(Bf
/Bi) = 200 µK.
(B -> D) The 2-gram silver or rhodium specimen of thin polycrystalline
foils, acting as the second nuclear stage, cools under the field
Bi = 8 T, by thermal conduction, to Tf
= 200 µK.
(D -> E) The specimen is adiabatically demagnetized from 8 T to 400
µT, whereby the spin assembly cools into the nanokelvin range, thermally
isolated by the slow spin-lattice relaxation ( 1 = 14 h) from the conduction electrons which are
anchored to 200 µK by the first nuclear stage at (C). By continuing
demagnetization of the specimen to zero field (not shown in the diagram), the
record temperature of 280 pK was reached in rhodium.
1 = 14 h) from the conduction electrons which are
anchored to 200 µK by the first nuclear stage at (C). By continuing
demagnetization of the specimen to zero field (not shown in the diagram), the
record temperature of 280 pK was reached in rhodium.
(E -> F) Finally, production of the negative temperature is achieved in
the system of silver or rhodium nuclei by flipping the 400-µT magnetic
field in about 1 ms. The rapid inversion results in some loss of polarization.
By continuing demagnetization to zero field, the record temperature of -750 pK
was reached in rhodium (not shown in the diagram).
(F -> G -> H -> A) The system starts to lose its negative
polarization, crossing in a few hours, via infinity, from negative to positive
temperatures.
(C -> A) The first nuclear stage warms slowly, under the 100 mT field, from
200 µK to 15 mK. A new experimental sequence can then be started.
The energy level diagram for an assembly of silver or rhodium nuclei is
illustrated schematically in the left part of Fig. 8; the spin I = 1/2,
so there are just two levels, corresponding to the nuclear magnetic
moment m parallel and antiparallel to the external magnetic field
B. The distribution of the nuclei among the Zeeman energy levels
is determined by the Boltzmann factor,
exp(m·B/kBT). At positive temperatures
the number of nuclei in the upper level, with m antiparallel to
B, is always smaller than in the lower. At the absolute zero,
all nuclei are in the ground level with m parallel to
B.
As the temperature is increased from T= +0, nuclei flip into the upper
energy band and, at T = + , there is an equal number of spins in both levels. When
the energy is increased further, the distribution of the nuclear spins can
still be described by the Boltzmann factor but now with T<0.
Finally, when approaching zero from the negative side, T-> -0,
eventually only the highest energy level is populated. Since heat is
transferred from the warmer to the colder part when two systems are brought
into thermal contact, negative temperatures are actually hotter than positive
ones. The crystalline lattice and conduction electrons cannot be cooled to a
negative temperature because in this case the energy spectrum has no upper
bound: at T<0 the energy of the system would be infinite.
, there is an equal number of spins in both levels. When
the energy is increased further, the distribution of the nuclear spins can
still be described by the Boltzmann factor but now with T<0.
Finally, when approaching zero from the negative side, T-> -0,
eventually only the highest energy level is populated. Since heat is
transferred from the warmer to the colder part when two systems are brought
into thermal contact, negative temperatures are actually hotter than positive
ones. The crystalline lattice and conduction electrons cannot be cooled to a
negative temperature because in this case the energy spectrum has no upper
bound: at T<0 the energy of the system would be infinite.
At T = +0, an isolated nuclear spin assembly thus has the lowest and,
at T = -0, the highest possible Helmholtz free energy. The tendency to
maximize energy, instead of minimizing it, is the basic difference between
negative and positive temperatures. In silver, the nearest-neighbor
antiferromagnetic Ruderman-Kittel exchange interaction, three times stronger
than the dipolar force, favors antiparallel alignment of the nuclear magnetic
moments and thus leads to antiferromagnetism when T>0. At
T<0, since the Helmholtz free energy now must be maximized, the very
same interactions produce ferromagnetic nuclear order.
Experimentally, population inversion from T>0 to T<0
is possible in silver and rhodium (see Fig. 8). This can be accomplished at
ultralow temperatures by reversing the magnetic field B quickly,
in a time t <<  2
= 10 ms, where
2
= 10 ms, where  2
is the spin-spin relaxation time, so that the nuclei have no chance to
rearrange themselves among the energy levels. In a certain sense, the system
passes from positive to negative temperatures via T = +
2
is the spin-spin relaxation time, so that the nuclei have no chance to
rearrange themselves among the energy levels. In a certain sense, the system
passes from positive to negative temperatures via T = + = -
= - , without crossing the absolute zero. Therefore, the
third law of thermodynamics is not invalidated. In copper
, without crossing the absolute zero. Therefore, the
third law of thermodynamics is not invalidated. In copper  2 is 100 times shorter than in
silver. Producing spontaneous nuclear ordering at T<0 is thus more
difficult in copper.
2 is 100 times shorter than in
silver. Producing spontaneous nuclear ordering at T<0 is thus more
difficult in copper.
To obtain nuclear temperatures in the nano- and picokelvin range, an
elaborate "brute force" cooling apparatus, with a dilution refrigerator and
two nuclear refrigeration stages in cascade, was employed. The cooling
procedure is described in detail with the aid of Fig. 8.
One of the difficult problems in these experiments is to measure the absolute
temperature of the nuclei. The second law of thermodynamics, T= Q/
Q/ S, was used directly, i.e., the nuclear
spin system was supplied with a small amount of heat
S, was used directly, i.e., the nuclear
spin system was supplied with a small amount of heat  Q and the ensuing entropy increase
Q and the ensuing entropy increase  S was calculated from the
measured decrease of polarization.
S was calculated from the
measured decrease of polarization.
NMR measurements showed that, instead of absorption as at T>0, the
spin system in silver was emitting energy when T<0. In rhodium
antiferromagnetic susceptibility was found at T>0 while, at
T<0, the ferromagnetic tendency changed at |T| = 5
nK into a tendency towards antiferromagnetism; phase transitions were not seen.
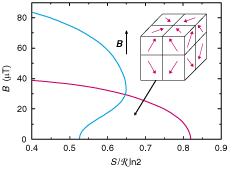
Fig. 9. Phase diagram of nuclear spins in silver for positive (blue)
and negative (red) absolute temperatures in the magnetic field (B)
versus reduced entropy (S/R ln2) plane. The ferromagnetic spin arrangement
at T<0 is illustrated in the insert: across a domain boundary
the tangential component of magnetization is continuous while the perpendicular
component changes sign.
It has been argued that negative temperatures are fictitious quantities
because they do not represent true thermal equilibrium in the sample
consisting of nuclei, conduction electrons, and the lattice. However, the
experiments on silver, in particular, show conclusively that this is not the
case. The same interactions produce ferro- or antiferromagnetic order in the
spin assembly, just depending on whether the nuclear temperature T<0
or T>0 (see Fig. 9).
Metals offer good models to investigate magnetism in general. The nuclei are
well localized, they are isolated from the electronic and lattice degrees of
freedom at low temperatures, and the interactions between nuclei can be
calculated from first principles. Therefore, these systems are particularly
suitable for testing theory against experiments. The realm of negative spin
temperatures offers new possibilities for studies of magnetism.







